VALUE PROPOSITION
Rabeximod; a first in class phosphoglycerate kinase 1 (PGK1) inhibitor for renal fibrosis; FSGS and AS treatment.
Anti-inflammation & fibrosis effect
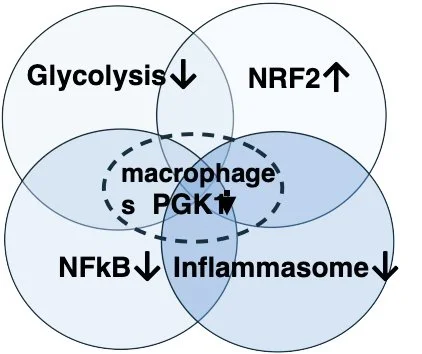
Targeting PGK1 reduce inflammation & fibrosis by metabolic alterations.
Inhibits macrophage driven fibrosis and inflammation.
Spares immune system.
Safe for infection sensitive patient population.
Favorable PK to inhibit kidney inflammation & fibrosis
Rabeximod ≥10-fold increase in kidney medulla & cortex, effectively targets kidney resident inflammatory- fibrosis driving macrophages.
Oral dosing, high ≥80% bioavailability, 2-4 days plasma half life.
Wide therapeutic index & excellent clinical safety profile, 312 patient cohort.
Rabeximod MoA
Executive Checklist
Clinical derisked asset – excellent safety and promising efficacy (312 patients)
CMC – GMP manufactured API is in stock for 500 patients
Long-term treatment secured – GLP-tox for 6 months performed
Drug Target and Mode of Action – Fibrosis /inflammation inhibition, macrophage targeting
Pharmacokinetics – advantageous for renal indications
IP approach established – OD indication allowing 15 years market exclusivity
Pipeline in a drug- expansion to other OD renal indications
Market & competition – conservative assumptions ~850 MUSD with minimal competition (+DKD market)
Experienced team - decades experience in biotech pharma building and drug development
Board of Directors – world-class excellence in biotech entrepreneurship
Venture capital backed
-
Capture the momentum of increasing understanding of the role of PGK1 in diabetic kidney disease progression and other glomerular nephropathies (IgAN, AS, FSGS).
Nephropathy treatment paradigms are shifting toward early combination therapies, providing an excellent opportunity for Rabeximod as a combination treatment with standard of care, based on its favorable safety profile and specific anti-fibrotic effects.
-
Rabeximod inhibits inflammation while sparing the immune system, a key value for infection-sensitive nephropathy patient populations.
Rabeximod targets PGK1, a recently validated and widely recognized driver of inflammation and fibrosis in glomerular nephropathies.
Favorable pharmacokinetics with kidney accumulation reduce systemic side effects. Oral administration and a long half-life provide a convenient treatment option.
-
De-risked project with near-time value inflection points.
Venture backed by Sound Bioventures and 3T Invest.
Operational excellence
Potential for State-of-the-art scientific/clinical advisory board in nephrology.